-
Posts
36,926 -
Joined
-
Last visited
-
Days Won
6
Content Type
Events
Forums
Downloads
Quizzes
Gallery
Blogs
Everything posted by TallGuyJohninBKK
-
Noted epidemiologist Katelyn Jetelina, author of the widely read Your Local Epidemiologist blog, has the following column explaining how two Trump Admin political appointees (Marty Makary and Vinay Prasad) with no expertise in vaccine matters totally bypassed both the FDA and CDC's own expert vaccine advisory panels in putting forward the FDA's new COVID vaccines policy... And why that's a problem. Covid-19 vaccines, what just happened at the FDA, and why it matters This is not how vaccine policy should be made. May 21,2025 "...beneath the surface, this move is deeply troubling. It bypasses the scientific systems built to answer these questions, replacing the public process in health policy with the opinions of two political appointees with chips on their shoulders. ... Yet, here we are. This decision was made without a [FDA advisory group] VRBPAC vote. No [CDC advisory group] ACIP meeting. No new data. No transparency or public discussion. Two FDA appointees decided the Covid-19 vaccine policy needed to change. Why that’s a big problem This matters for several reasons: It undermines the evidence-based process. Makary and Prasad—neither of whom is a vaccinologist or has experience leading vaccine policymaking—circumvented the rigorous, transparent system in place. This was neither collaborative nor grounded in new evidence. There was no urgent reason to bypass that process. VRBPAC meets this Thursday, just two days after this announcement. (more) https://yourlocalepidemiologist.substack.com/p/covid-19-vaccines-what-just-happened
-
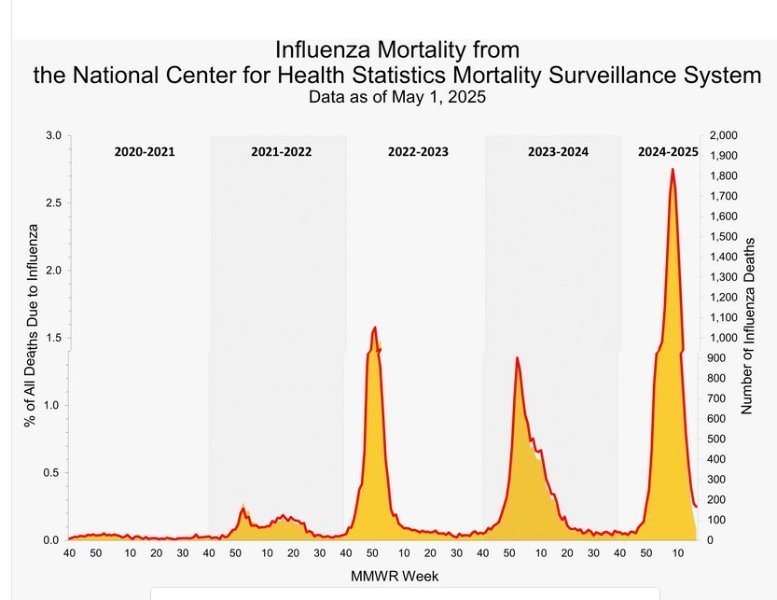
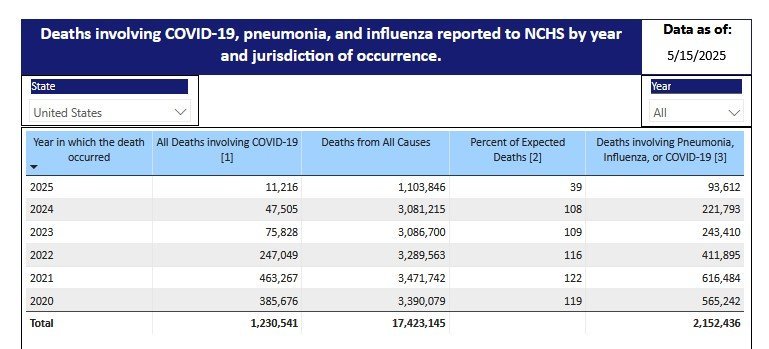
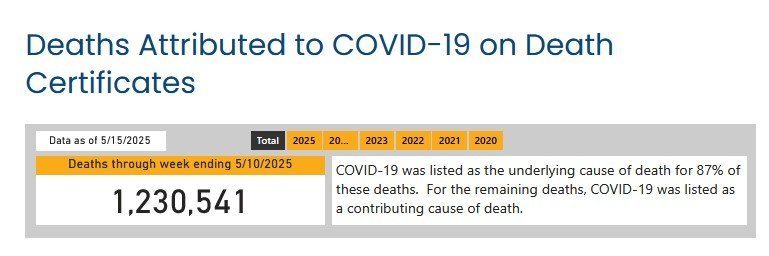
As usual, you're throwing out unsourced and unsubstantiated claims that aren't supported by the facts: The CDC estimates there have been 26,000 U.S. flu deaths thus far this season vs. 11,216 COVID deaths since January. This year, as shown below, was a very bad season for flu deaths compared to recent years. Want to avoid dying from the flu (and COVID)... Get vaccinated! CDC on this year's flu cycle: "This season is classified as a high severity season overall and for all age groups (children, adults, older adults) and is the first high severity season since 2017-2018. CDC continues to recommend that everyone ages 6 months and older get an annual flu vaccine as long as influenza viruses are circulating.1" https://www.cdc.gov/fluview/surveillance/2025-week-17.html
-
The WHO advisory group already met and announced in recent days their recommendations for updating COVID vaccines.... May 16, 2025 "The World Health Organization (WHO) Technical Advisory Group on COVID-19 vaccine composition, after meeting earlier this month, today released its recommendations for updated vaccines, which say the current monovalent JN.1 or KP.2 strains are still appropriate, but monovalent LP.8.1 is a suitable alternative. Over the last 2 years, after examining the latest data on virus changes and response to current vaccines, the group has been weighing in on strain recommendations twice a year, once in the spring and once in December. The WHO’s latest recommendations come ahead of a meeting of the Food and Drug Administration (FDA) vaccine advisory group on May 22, which will discuss the make-up of COVID vaccines for use in United States during the fall and winter months. Current US vaccines include the KP.2 antigen. Also, the WHO recommendations come as a few countries in Asia report rising COVID activity." (more) https://www.cidrap.umn.edu/covid-19/who-advisers-say-current-strains-ok-covid-vaccine-production
-
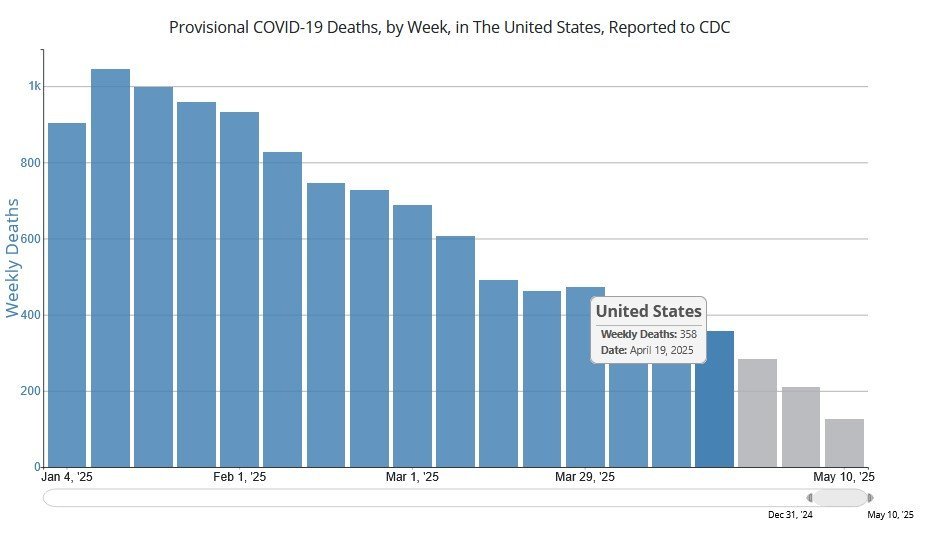
Now is the relative quiet season in the U.S. for COVID, with the infection peaks usually coming around the end of the year each year, with weekly COVID deaths in the U.S. peaking at more than 1,000 per week last January (as shown below). And yet, the latest reports from the CDC say that COVID deaths in the U.S. (those where COVID is the underlying or contributing cause) even now in the quiet season are running more than 350 people each and every week. That's a lot of COVID deaths for a disease and threat that the resident anti-vaxers here like to dismiss and claim is a non-serious infection. * the three gray colored columns at the right of the above chart reflect incomplete data, and thus are colored differently to reflect that. https://covid.cdc.gov/covid-data-tracker/#trends_weeklydeaths_select_00
-
FDA advisory panel debates COVID vaccine recipe as questions swirl about fall shots May 22, 2025 WASHINGTON (AP) — Government advisers are meeting Thursday to decide if COVID-19 vaccines need updating to improve protection this fall and winter — even as a new Trump administration policy has thrown into question who may be eligible for a shot. ...the FDA said routine approval of COVID-19 boosters will be limited to seniors and to younger people who are at high risk of severe infection. Manufacturers will need to do new studies to show whether seasonal shots still benefit healthy people younger than 65. That raises big implications for next fall’s vaccination campaign, with uncertainty over whether healthy people still could get a vaccine even if it’s not recommended for them — or whether insurers will keep paying for the shots for everyone. Nor is it clear what the policy means for babies who have never been vaccinated. (more) https://apnews.com/article/fda-vaccines-covid-boosters-kennedy-244bdc80f825f953782d35f68798d885
-
The FDA’s New Covid Vaccine Policy Is Clear as Mud Healthy agency leaders’ promises of transparency and choice fall short in first big test May 22, 2025 ... "Here’s what we know: The FDA is adopting a risk-based strategy for approving new Covid vaccines that will limit their use to people 65 and older or anyone with a health condition that puts them at risk for severe disease — a group Prasad and Makary estimate accounts for about 100 million Americans. The new policy keeps the current standards for greenlighting vaccines, meaning pharmaceutical companies need only to produce data showing theirs prompts people to produce antibodies against the virus. For healthy adults and children, new vaccines, including boosters updated to match circulating strains of the virus, seem to be on ice. FDA officials will require drug manufacturers to run studies proving their vaccines are better than a placebo in preventing symptomatic infections in people ages 50 to 64 — a group Prasad said was chosen because health agencies around the world express the most disagreement over the benefits of vaccination for the demographic. They said companies can choose to pursue studies that demonstrate their drugs effects in other groups. The future of pediatric Covid vaccines seems particularly foggy, since updated shots also function as the primary series for unvaccinated children. While billed as “a balance of regulatory flexibility and a commitment to gold-standard science,” the policy seems designed to put the vaccines out of reach. (more) Bloomberg News https://archive.ph/a2bqs
-
Older adults can continue getting covid boosters, but not everyone who wants them can May 22,2022 ... The framework, unveiled on Tuesday in a New England Journal of Medicine article co-authored by FDA commissioner Marty Makary and center director Vinay Prasad, is decidedly better than my worst fears. It preserves vaccine access for people most vulnerable to covid-19. However, it takes away the freedom of other groups to choose to protect themselves and raises questions about how available these shots will be going forward. The most important part of the FDA’s announcement is that the agency is expected to continue approving boosters for people 65 and older based on immune response data. This is terrific news; it means manufacturers won’t have to conduct time- and resource-intensive randomized controlled trials when new variants arise. This alleviates my concern that there will be long delays that will leave the most vulnerable Americans unprotected. Older adults should also be able to continue getting covid boosters. This is a group that clearly benefits from added protection. The Centers for Disease Control and Prevention reports that adults 65 and older now account for 68 percent of all covid-associated hospitalizations and, between September 2023 and August 2024, nearly 90 percent of all deaths. (more) Washington Post https://archive.ph/jXGQo
-
I know there's a process to file a regular will (not a living will) at the amphur office where one has residency. It's often called an amphur will. But I'm not aware of any comparable process that involves filing a living will (advance medical care directive) with one's local amphur office... As it's the hospital and doctors you end up at that need to have the executed living will document. In your post above, were you referring to a living will (advance medical care directive) or a traditional will that spells out what happens to your assets after your death?
-
Except what you claim above re without usual testing simply isn't/wasn't true, as explained below from USA Today Fact Check and Boston University COVID vaccine myths article, among others: "Having a running start does not mean the testing process was accelerated, however. Hotez ... stated the vaccine still underwent testing among a large group of human volunteers, even more than a typical trial with over 30,000 to 60,000 people. (emphasis added) What accelerated the vaccine process was manufacturing. "The two accelerants are doing the manufacturing of risk (scaling up manufacturing based on the assumption the vaccine will work, also called at-risk manufacturing) and manufacturing the vaccine in parallel with clinical trials. That's new because we usually wait for the phase three results," he said. https://www.usatoday.com/story/news/factcheck/2021/01/21/fact-check-covid-19-vaccine-nearly-20-years-making/3873247001/ Boston University - 2021: Myths vs. Facts: Making Sense of COVID-19 Vaccine Misinformation When so much wrong information is readily available, convincing people to get vaccinated has proven to be a huge challenge ... MYTH: The COVID vaccines were not rigorously tested, which is why they have only emergency authorization approval and not full Food and Drug Administration approval. (Update: Pfizer’s vaccine received full FDA approval on August 19) FACT: “Vaccine developers didn’t skip any testing steps, but conducted some of the steps on an overlapping schedule to gather data faster.”—Johns Hopkins Medicine (emphasis added) Davidson Hamer, a faculty member of BU’s School of Public Health: "The development was more rapid than many other vaccines. But it used the same process of phase one and phase two trials following appropriate safety measures. Stage three trials were large-scale trials done rigorously with very clear outcome definitions. The safety measures and approaches taken are standard for clinical trials. They just did it more rapidly than usual." https://www.bu.edu/articles/2021/myths-vs-facts-covid-19-vaccine/
- 176 replies
-
- 26
-

-

-

-

-

-

-
I need to do a living will as well... In the past, when I've dipped my toes into those waters, it seemed like at least SOME private hospitals in BKK (Bummers) wanted you to pay for a consult with their elder care doctors and then have you/them fill out the HOSPITAL'S specific version of a living will document. And it wasn't at all clear, if you just randomly showed up for care, if they'd accept/honor a generic version not done in their own format. The potential problem, of course, if that one never knows potentially many years in advance just what hospital one might end up in when the need arises.... If anyone has any direct experience with the issue of private hospitals in BKK insisting on their version of private wills, feedback would be much appreciated. Really, one generic version that complies with the Thai legal requirements ought to be sufficient.
-
Congrats on making it to the big 8-0!!! 🙂
-
U.S. FDA Approves BLA for Novavax's COVID-19 Vaccine May 19, 2025 GAITHERSBURG, Md., May 19, 2025 /PRNewswire/ -- Novavax, Inc. (Nasdaq: NVAX) today announced that the U.S. Food and Drug Administration (FDA) has approved the Biologics License Application (BLA) for Nuvaxovid™ for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in adults 65 years and older and individuals 12 through 64 years who have at least one underlying condition that puts them at high risk for severe outcomes from COVID-19 (e.g. asthma, cancer, diabetes, obesity, smoking). "Today's approval solidifies a pathway for Americans aged 65 and older and those aged 12 through 64 with an underlying condition that puts them at high risk for severe outcomes from COVID-19 to have access to a protein-based, non-mRNA COVID-19 vaccine," said John C. Jacobs, President and Chief Executive Officer, Novavax. "Market research and U.S. CDC statistics indicate that older individuals and those with underlying conditions are the populations most likely to seek out COVID-19 vaccination seasonally. This significant milestone demonstrates our commitment to these populations and is a significant step towards availability of our protein-based vaccine option." ... Novavax expects to be ready for the commercial delivery of the 2025-2026 COVID-19 vaccine formula in the U.S. this fall in partnership with Sanofi, pending strain recommendation at the FDA Vaccines and Related Biological Products Advisory Committee meeting on May 22, 2025. Nuvaxovid has been available for use in the U.S. under Emergency Use Authorization since July 2022 and has full market approvals in the European Union, United Kingdom, Japan, Canada, Australia, Taiwan and Singapore. (more) https://ir.novavax.com/press-releases/2025-05-19-U-S-FDA-Approves-BLA-for-Novavaxs-COVID-19-Vaccine
-
World agrees pandemic accord for tackling outbreaks of disease Hailed as ‘a victory for public health,’ the agreement aims to build on the lessons of Covid-19 and protect the globe from pathogenic threats ... In order to reach the agreement this week, some key points of contention have been pushed back for later talks. The issue of pathogen access and benefit sharing (Pabs) – or what countries can expect, in terms of access to vaccines and treatments, in return for sharing data on any novel bugs emerging in their territory – will be governed by an annexe to the treaty, to be negotiated over the next 12 months. The Independent Panel for Pandemic Preparedness and Response recommended an agreement of this kind four years ago after reviewing the international response to Covid-19. The panel co-chair, former prime minister of New Zealand Helen Clark, said the agreement should be considered “a foundation from which to build, starting today”. ... The agreement will not open for signatures until the Pabs annexe is completed. It will then come into force after at least 60 countries have signed. However, it is already being seen as a key achievement for the WHO at a time of crisis, with lower funding after the US withdrew necessitating dramatic cuts. (more) https://www.theguardian.com/global-development/2025/may/20/world-agrees-pandemic-accord-for-tackling-outbreaks-of-disease-who-covid
-
20 May 2025 Agreement’s adoption follows three years of intensive negotiation launched due to gaps and inequities identified in national and global COVID-19 response. Agreement boosts global collaboration to ensure stronger, more equitable response to future pandemics. Member States of the World Health Organization (WHO) today formally adopted by consensus the world's first Pandemic Agreement. The landmark decision by the 78th World Health Assembly culminates more than three years of intensive negotiations launched by governments in response to the devastating impacts of the COVID-19 pandemic, and driven by the goal of making the world safer from – and more equitable in response to – future pandemics. “The world is safer today thanks to the leadership, collaboration and commitment of our Member States to adopt the historic WHO Pandemic Agreement,” said Dr Tedros Adhanom Ghebreyesus, WHO Director-General. “The Agreement is a victory for public health, science and multilateral action. It will ensure we, collectively, can better protect the world from future pandemic threats. It is also a recognition by the international community that our citizens, societies and economies must not be left vulnerable to again suffer losses like those endured during COVID-19.” Governments adopted the WHO Pandemic Agreement today in a plenary session of the World Health Assembly, WHO’s peak decision-making body. The adoption followed yesterday’s approval of the Agreement by vote (124 in favour, 0 objections, 11 abstentions) in Committee by Member State delegations. (more) https://www.who.int/news/item/20-05-2025-world-health-assembly-adopts-historic-pandemic-agreement-to-make-the-world-more-equitable-and-safer-from-future-pandemics
-
Nuvaxovid becomes the only non-messenger RNA COVID-19 vaccine approved by the FDA The FDA has approved Novavax’s protein-based COVID-19 vaccine, giving some Americans another option besides messenger RNA vaccines to prevent SARS-CoV-2 infection. The approval came with restrictions that the other COVID-19 vaccines do not have, reducing the number of people who will be eligible to receive the shot, branded as Nuvaxovid, which had been available under an emergency use authorization since 2022. The FDA restricted approval of the single-dose adjuvanted vaccine to people aged 65 years or older, and adolescents or adults aged 12 to 64 years with at least one risk factor for severe COVID-19. The agency also will require Novavax to conduct post-approval studies to evaluate the occurrence of two heart conditions, myocarditis and pericarditis, rare side effects of COVID-19 vaccines. (more) https://www.healio.com/news/infectious-disease/20250519/fda-approves-novavaxs-covid19-vaccine-with-restrictions
-

Panicked Trump Lashes Walmart for Telling Truth on Tariffs
TallGuyJohninBKK replied to BLMFem's topic in Political Soapbox
Anything that doesn't line up with his nonsense narratives has to be attacked and the people voicing those opinions muzzled -- even if they happen to be accurate and true. Truthfulness has never been a particular attribute of Trumpworld or MAGA.- 62 replies
-
- 13
-

-

-

-

-

-

Panicked Trump Lashes Walmart for Telling Truth on Tariffs
TallGuyJohninBKK replied to BLMFem's topic in Political Soapbox
The imbecile has been doing a whole lot of frustrated "lashing out" lately... His list of perceived personal grievances and grudges is long and ill-suited to anyone who calls themself the "President of the United States." But as the saying goes, "it is what it is..." -

FREE Covid vax still happening?
TallGuyJohninBKK replied to unblocktheplanet's topic in COVID-19 Coronavirus
The Thai Travel Clinic at Mahidol University near the Victory Monument BTS station is listing availabiliity of the COVID JN.2 version vaccine for their price of 1,762 baht. https://www.thaitravelclinic.com/cost.html The Thai Red Cross Clinic (Travel and Immunization Clinic) in the past also had the COVID vaccine at a similar price, though you'd have to check with them about their current status. Their vaccines list on their website says they have it available, but the posted list seems to be from 2021 and hasn't been updated. https://www.facebook.com/QSMITRCS/posts/pfbid02X2MW3goL9xzNmdBKohKu91V1NZF4fcrkZPYGC2NZqoPXSyybZ5Uke6tLGr7iExW8l https://saovabha.org/service_saovabha/Our-Clinic https://saovabha.org/home Contact Us 1871 Rama 4 Road, Pathumwan, Bangkok 10330 info[at]saovabha.org 0 2252 0161 Office Hours Monday – Friday 08.30 – 16.30 hrs. clinic opens on Monday-Friday : 1.00 PM - 4.00 PM Saturday Sunday and Public Holiday : closed ------------------------------------- Last time I checked in BKK, both Bumrungrad and Praram 9 private hospitals were providing COVID vaccines as well, but at substantially higher prices. ------------------------------------- The former free COVID vaccination clinics were sponsored by / run by the Thai Government and its Ministry of Public Health. But I've heard/seen nothing from them lately on that subject. The MOPH / Department of Disease Control does have an English-speaking call center reachable via phone 1422 that in the past has had info about the extent of COVID vaccines available around BKK, both from public and private sources (though their private source details were not always accurate/up-to-date). Believe the hotline is staffed M-F 8 am to 8 pm. The last time I checked more than a year ago, MOPH was providing COVID vaccines on just certain days at various of their community public health clinics scattered around BKK, and at some of their public hospitals... But you'd have to check with MOPH/DDC about whether those offerings have lapsed or are still continuing (albeit unpublicized). Anything you find in the way of results, please do post that info back here for others to see. Thx. -

US Charles Schwab Residency?
TallGuyJohninBKK replied to BKKKevin's topic in Jobs, Economy, Banking, Business, Investments
Thanks for the above. Don't think I'd ever seen / noticed the #2 item you quoted above. Very helpful, as regards the Schwab Intl. account and the U.S. tax implications for it! -

US Charles Schwab Residency?
TallGuyJohninBKK replied to BKKKevin's topic in Jobs, Economy, Banking, Business, Investments
I think the gift tax scenario @Yumthai is talking about is the up to 40% U.S. gift tax that comes into play where a NRA (in this case Thai spouse) inherits U.S. brokerage assets from an American spouse accountholder.